Intertek offers bioinformatic assessments to assist companies with characterizing and demonstrating the safety of microorganisms and microbial-derived products.
New technology is allowing companies to use microorganisms and microbially-derived products to meet the societal shift towards a sustainable food supply.
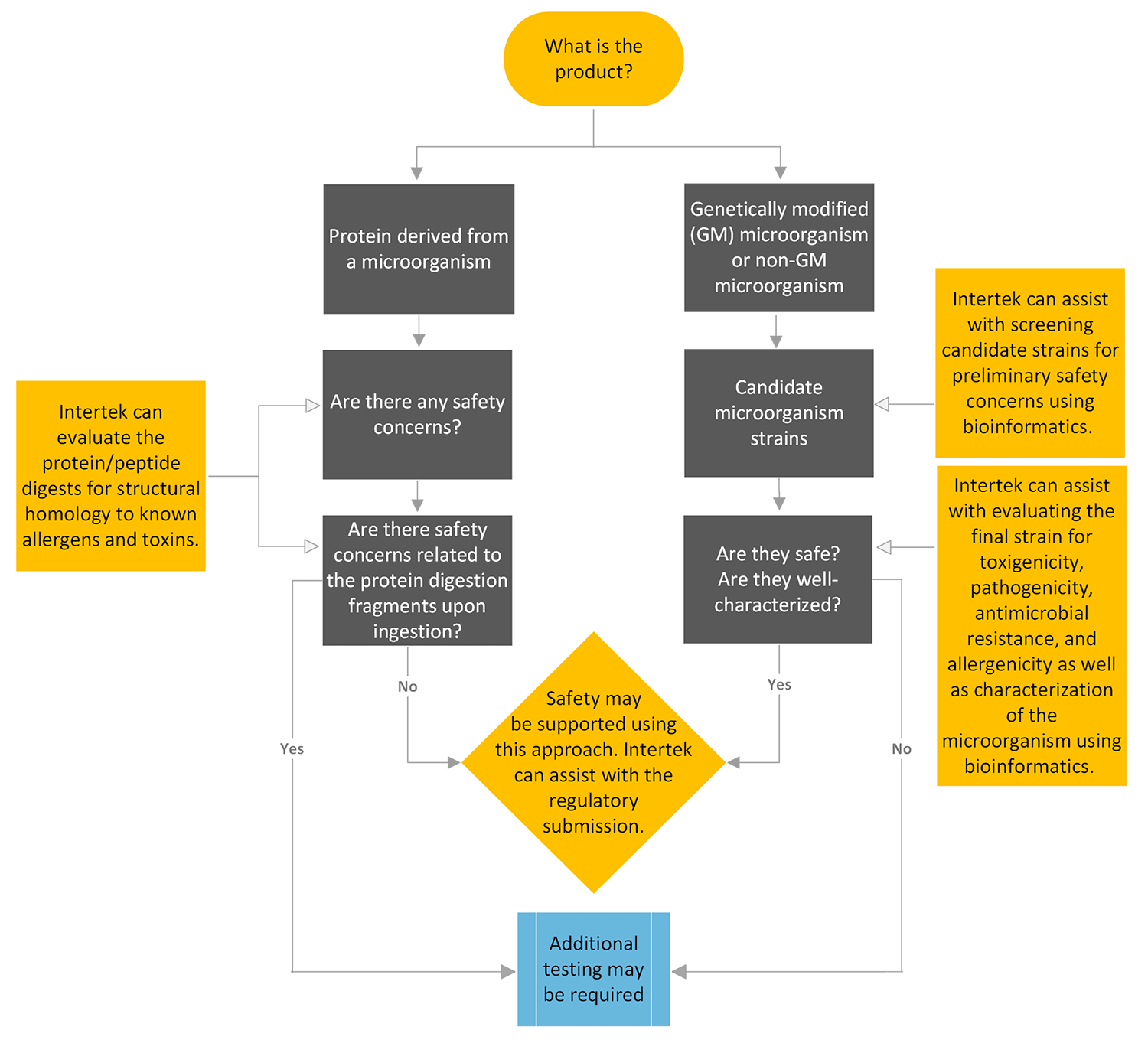
In the food industry, regulatory approval of these products can be supported using a tiered approach, using bioinformatics—an innovative safety evaluation paradigm—in lieu of animal studies. This is urgently needed in a world that demands more sustainable solutions.
Regulators have recognized the potential for bioinformatic evaluations to address safety concerns such as antimicrobial resistance, pathogenicity, toxigenicity, and allergenicity, and to better characterize these products. However, few systematic approaches have been developed.
The Intertek Advantage
With over 30 years of scientific and regulatory expertise, Intertek provides customers with industry-leading risk assessments and scientific evaluation services. Our demonstrated expertise in regulatory approval of food ingredients in multiple jurisdictions makes Intertek the chosen compliance partner for the world’s leading companies.
Intertek's track record of success in regulatory approvals, and our strong relationships with regulatory authorities, provide the ideal foundation for companies applying for innovative compliance processes and techniques including bioinformatic assessments.
Intertek’s bioinformatic assessment service is a systematic approach that provides a tiered risk-assessment methodology for the safety evaluation and characterization of genetically modified (GM) and non-GM microorganisms, and products derived from such microorganisms.
Through a tiered approach, Intertek supports companies by:
 |
|
Intertek’s bioinformatic assessment service is a unique offering that follows regulatory guidelines, where applicable, and utilizes the latest scientific advancements and knowledge to offer our customers cost-effective compliance programs and a decreased time to market.
Safety Evaluation Procedure for Microbial Products and Microbially-Derived Products
Related Services
Generally Recognized as Safe (GRAS) Services
Food Additives and Food Enzymes Services
Novel Food & Food Additive Services
Food Microbiological Testing Services
Food New Product Development
Food Biotechnology Services
Computational Predictive Toxicology Modeling Services
Canadian New Substances Notification (NSN) Regulations Services


